Cancer, the second leading cause of death worldwide, presents one of the principal healthcare challenges of our time.
Novocure was founded in 2000 to develop Tumor Treating Fields, a new approach to treating cancer that targets the electrical properties of proteins to kill dividing cancer cells. Today, we are a global oncology company with two FDA-approved indications, a third indication under FDA review and four additional phase 3 clinical programs. At Novocure, delivering better outcomes for patients with some of the most aggressive forms of cancer drives our work each day.
2018 was a particularly productive year
We entered 2018 with two clear priorities: first, to drive commercial adoption of Optune for the treatment of glioblastoma (GBM); and second, to advance our clinical pipeline. We finished the year with growth in our GBM business globally and tangible progress in our clinical development programs. Highlights included presenting our STELLAR data in malignant pleural mesothelioma (MPM), enrolling the first patient in our phase 3 pivotal PANOVA-3 trial for pancreatic cancer, signing our collaboration agreement with Zai Labs to bring Tumor Treating Fields to China, receiving national reimbursement in Sweden, and finishing the year with almost $250 million in annual revenue.
We are grateful for the trust and confidence of our patients, prescribers and shareholders. I am particularly grateful for the dedication and hard work of my Novocure colleagues who make our progress possible.
Driving commercial adoption of Optune
Optune is proven to provide quality, long-term survival for patients with newly diagnosed GBM. Since our first FDA approval, we have made significant investments to establish our commercial infrastructure around the world. To date, we have treated more than 10,000 GBM patients and have posted 16 consecutive quarters of active patient growth since the initial presentation of our EF-14 trial data in newly diagnosed GBM. In 2018, prescriptions for patients with newly diagnosed GBM grew 40 percent year-over-year.
“Today, we are a global oncology company with two FDA-approved indications, a third indication under FDA review and four additional phase 3 clinical programs.”
total perscriptions
0%
(USD in thousands)
total active patients
0%
(USD in thousands)
total operating revenue
0%
(USD in thousands)
In the United States, total prescriptions grew more than 20 percent in 2018 compared to the prior year. We continue to focus the tremendous efforts of our sales and marketing teams on increasing oncologists’ confidence and comfort describing our clinical benefits to patients and on increasing adoption in the community setting, particularly among radiation oncologists. In the U.S., we also rolled out several direct-to-patient initiatives, including groundbreaking Facebook Live programs, an expanded patient ambassador program, and an updated direct-to-patient campaign highlighting Optune’s long-term survival and quality-of-life data.
In EMEA, we focused primarily on the German market. We were also active in Austria, Switzerland and Israel. Total prescriptions grew 14 percent in 2018 compared to the prior year. Notably, net revenues grew more than 70 percent year-over-year, driven by a meaningful improvement in the reimbursement approval rate of individual claims in Germany and by national reimbursement in Austria. In January 2019, we announced receipt of national reimbursement in Sweden. Our commercial launch in Sweden is underway.
2018 was our first full year of commercial activity in Japan. We signed contracts with more than 100 hospitals to provide Optune; we filled 162 prescriptions and delivered more than $6.3 million in net revenues. We remain enthusiastic about the opportunity to grow adoption of Optune in this key market.
In September 2018, we announced a strategic collaboration with Zai Lab including a license agreement for Tumor Treating Fields in Greater China and a clinical development cooperation agreement. Zai Lab shares our patient-forward vision and our business values. Zai hit the ground running and already has begun to treat GBM patients in Hong Kong.
As we enter 2019, we believe there is significant opportunity for further growth in our GBM business across geographies. Our teams continue to focus on driving adoption through physician education, clinical research and, where permitted, direct-to-patient outreach. We know there are many more patients who can benefit from Optune than are currently on therapy.
Advancing the pipeline
Tumor Treating Fields is a physics-based therapy that is broadly applicable to solid tumor cancers. The application of electric fields to attract and repel charged proteins during cell division has demonstrated an effect in every cancer cell line tested. The mechanism of action is independent of genotype, phenotype or micro-environment. It only requires the appropriate placement of transducer arrays to create electric fields tuned to specific frequencies within targeted cancer cells.
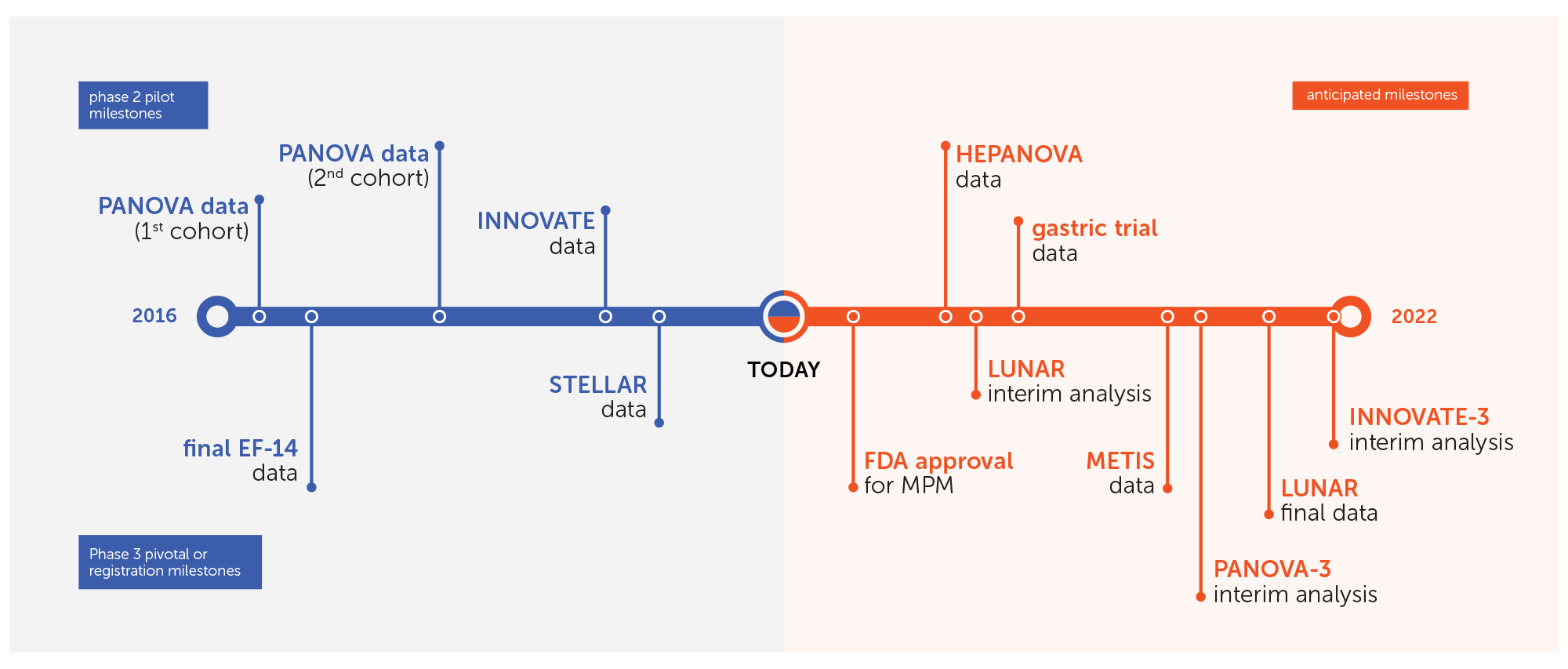
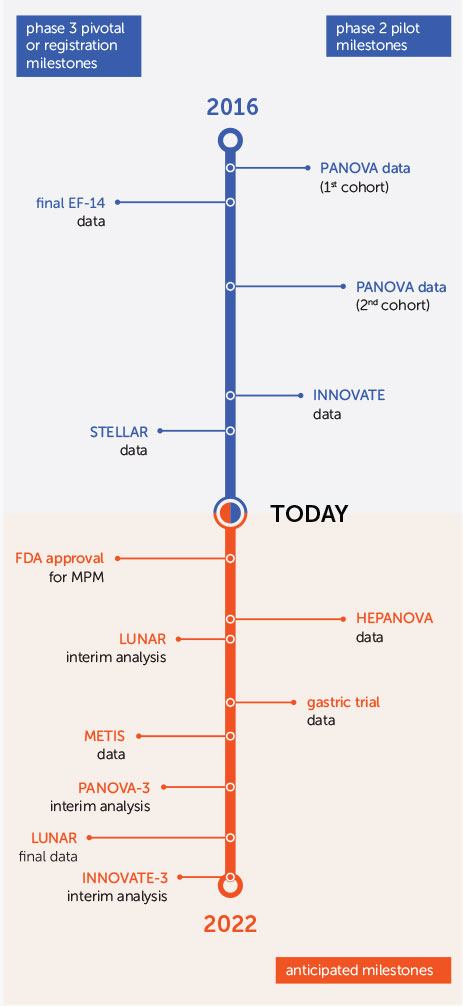
Since our initial public offering in 2015, we have presented successful phase 2 pilot data in multiple solid tumor indications. These clinical milestones have created the foundation for a drumbeat of late-stage clinical and regulatory milestones expected over the next three years.
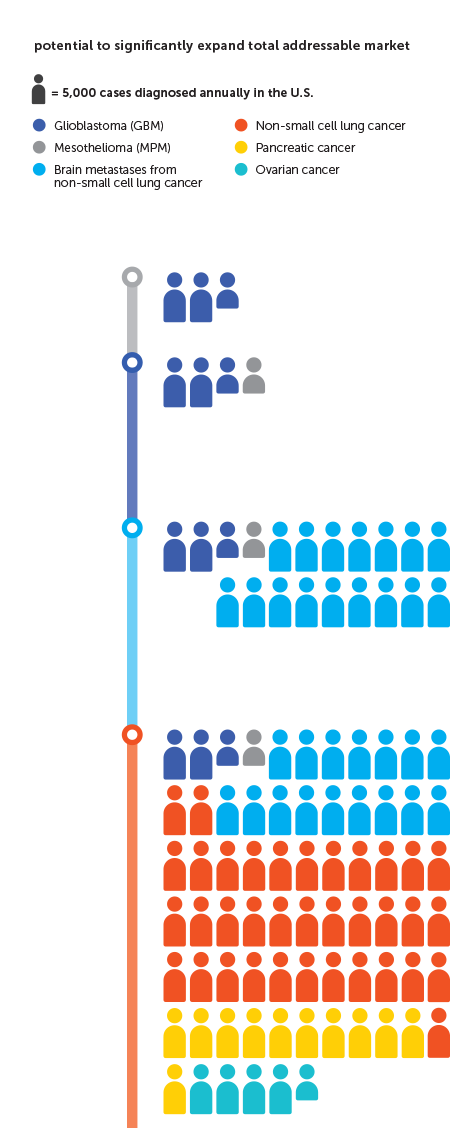
In 2018, we presented data from our STELLAR registration trial studying Tumor Treating Fields in unresectable MPM. Patients who received Tumor Treating Fields with pemetrexed and cisplatin or carboplatin experienced a median overall survival of 18.2 months. As expected, there was no increase in systemic toxicity when Tumor Treating Fields was added to the standard of care chemotherapy regimen.
MPM is one of the most aggressive forms of cancer. There has not been significant progress in treating MPM since the approval of pemetrexed more than 15 years ago. Our STELLAR data have been submitted to the FDA for marketing approval under the Humanitarian Device Exemption (HDE) pathway provided by our Humanitarian Use Device (HUD) designation. We are in constructive, ongoing discussions with the FDA and hope to receive approval for MPM in 2019.
We continue to enroll patients in our phase 3 pivotal trials in brain metastases, non-small cell lung cancer and pancreatic cancer, and initiated our phase 3 pivotal trial in ovarian cancer in March 2019. We are also recruiting a phase 2 pilot trial in liver cancer and are working with Zai Lab to initiate a phase 2 pilot trial in gastric cancer in China.
We continue to perform basic research on Tumor Treating Fields as do members of the independent scientific community. In 2018, we developed significant new insights that may enable us to further improve efficacy beyond what we have seen in our trials, to date.
A post-hoc analysis of the EF-14 data showed that more time on Optune predicted increased survival of GBM patients. In 2018, a separate post-hoc analysis showed that higher levels of energy (mW/cm3) at the tumor bed predicted increased survival of GBM patients, independent of time on therapy. For Optune, dose density can now be defined as time on therapy times the energy delivered, the cumulative energy delivered.
With this knowledge, our engineering efforts are now geared toward Optune system improvements to improve efficacy and extend survival. We have developed novel mapping algorithms to optimize treatment planning and our teams are working to incorporate these algorithms into a second generation NovoTAL system. We are also working to develop second generation transducer arrays to improve patient comfort and convenience and to maximize the energy delivered to the tumor bed and surrounding tissues.
Creating shareholder value
Novocure is a high-growth oncology company with significant growth opportunities remaining in GBM and in much more prevalent cancers. Our balance sheet is strong. We ended 2018 with $246 million cash, cash equivalents and short-term investments on hand, and largely have been able to fund our investments in additional indications and Optune system technology improvements with cash flow from the GBM business.
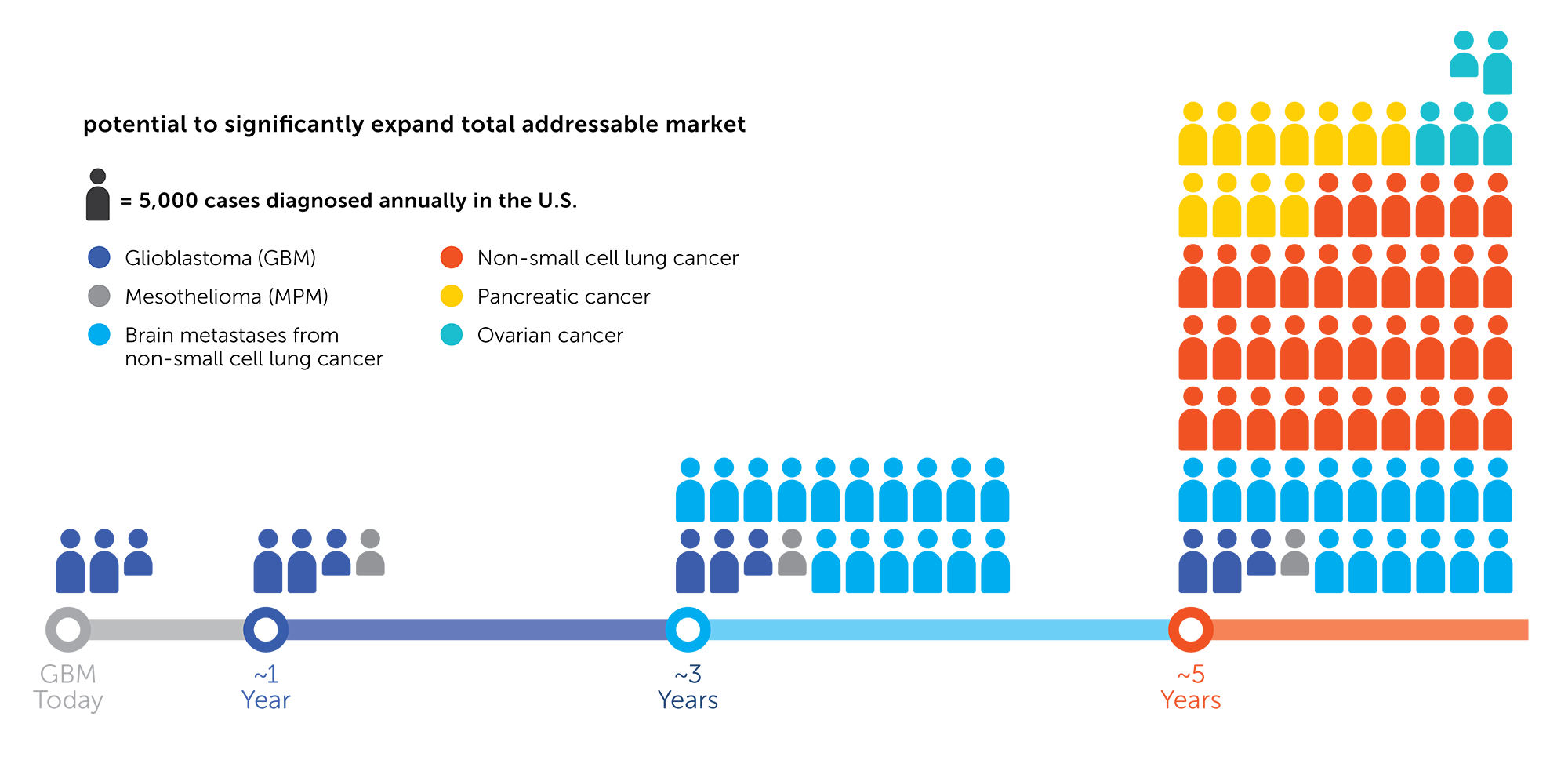
While GBM alone provides an important opportunity for Novocure to improve patient outcomes and grow revenue, it represents the tip of iceberg for the company. We believe the total potential addressable market for Optune in additional cancer indications is exponentially larger than the opportunity we see today in GBM. We believe our extensive IP portfolio and our extended cash runway provide the stability and flexibility to execute our core strategies.
Investing in our people and culture
From the day Professor Yoram Palti founded Novocure in 2000, we have maintained an encompassing effort to ensure every employee shares his patient-forward vision. Now, as a 600-plus-person organization, we have recommitted to ensure a shared focus on our core mission to extend survival in some of the most aggressive forms of cancer by developing and commercializing Tumor Treating Fields — to extend life without interfering with quality of life. Looking ahead, I am enthusiastic and energized about our opportunities as we work to accomplish our mission.
Thank you for your continued support.
Bill Doyle,
Executive Chairman
