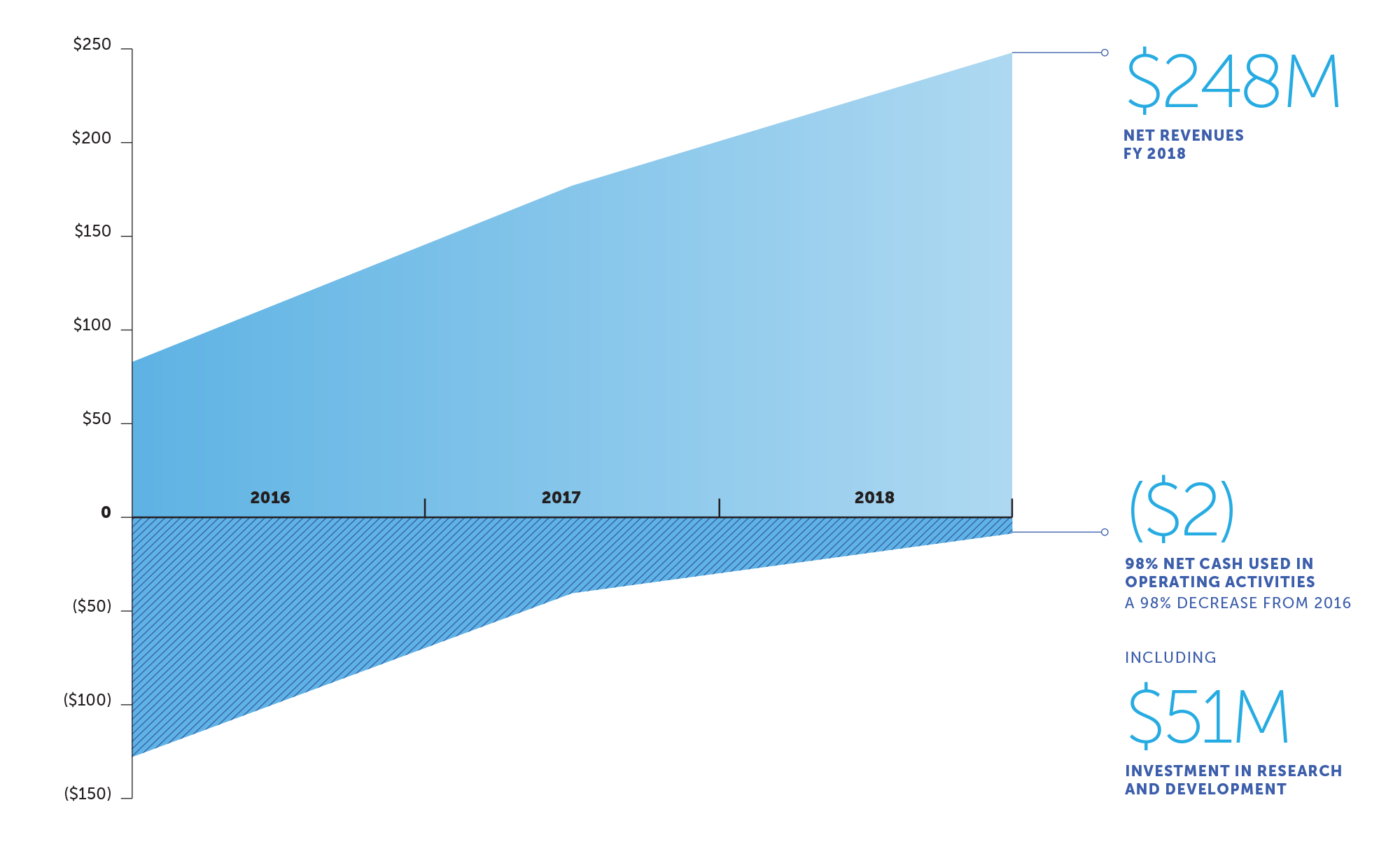
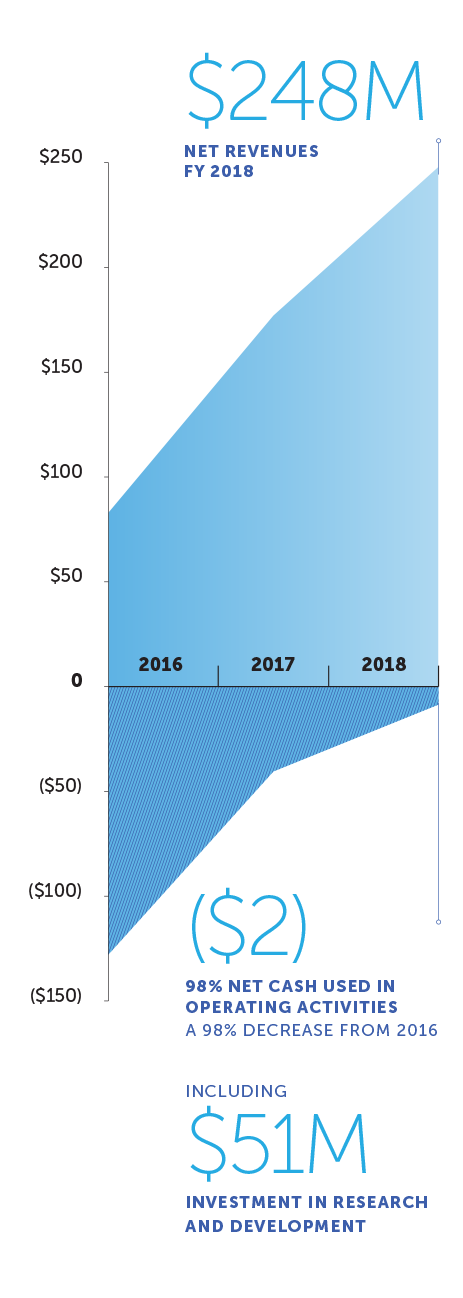
Cash flow from the glioblastoma business is funding increased investments in R&D
global net revenues (USD in millions)
Our balance sheet is strong
cash, cash equivalents and
short-term investments
(USD in millions)
consolidated statement of operations
| Year ended December 31, | U.S. dollars in thousands | 2018 | 2017 | 2016 | 2015 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Net revenues | $ 248,069 | $ 177,026 | $ 82,888 | $ 33,087 | |||||||
| Cost of revenues | 80,048 | 55,609 | 39,870 | 20,610 | |||||||
| Impairment of field equipment | – | – | 6,412 | – | |||||||
| Gross profit | 168,021 | 121,417 | 36,606 | 12,477 | |||||||
| Research, development and clinical trials | 50,574 | 38,103 | 41,467 | 43,748 | |||||||
| Sales and marketing | 77,663 | 63,528 | 59,449 | 38,861 | |||||||
| General and administrative | 73,456 | 59,114 | 51,007 | 33,864 | |||||||
| Total operating expenses | 201,693 | 160,745 | 151,923 | 116,473 | |||||||
| Operating income (loss) | (33,672) | (39,328) | (115,317) | (103,996) | |||||||
| Financial expenses, net | (12,270) | (9,169) | (6,147) | (3,151) | |||||||
| Income (loss) before income taxes | (45,942) | (48,497) | (121,464) | (107,147) | |||||||
| Income taxes | (17,617) | (13,165) | (10,381) | 4,434 | |||||||
| Net income (loss) | $ (63,559) | $ (61,662) | $ (131,845) | $ (111,581) | |||||||
| Basic and diluted net income (loss) per ordinary share | $ (0.69) | $ (0.70) | $ (1.54) | $ (3.67) |
consolidated balance sheet
| U.S. dollars in thousands | 2018 | 2017 | 2016 | 2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cash and cash equivalents | $ 140,622 | $ 78,592 | $ 99,780 | $ 119,423 | |||||||
| Short-term investments | 105,256 | 104,719 | 119,854 | 150,001 | |||||||
| Total assets | 339,793 | 265,298 | 282,081 | 307,336 | |||||||
| Working capital | 256,809 | 194,932 | 224,991 | 265,277 | |||||||
| Current liabilities | 64,560 | 50,202 | 36,882 | 28,627 | |||||||
| Long-term liabilities | 162,974 | 101,532 | 102,854 | 27,889 | |||||||
| Total shareholders' equity | 112,259 | 113,564 | 142,345 | 250,820 |
condensed cash flow
| U.S. dollars in thousands | 2018 | 2017 | 2016 | 2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Net cash provided by (used in) operating activities | $ (1,865) | $ (33,134) | $ (107,592) | $ (99,884) | |||||||
| Net cash provided by (used in) investing activities | (5,493) | 8,628 | 12,996 | (115,269) | |||||||
| Net cash provided by (used in) financing activities | 69,369 | 5,168 | 75,124 | 276,989 | |||||||
| Effect of exchange rate differences on cash and cash equivalents | 27 | 8 | 10 | – | |||||||
| Net increase (decrease) in cash, cash equivalents and restricted cash | 62,038 | (19,330) | (19,462) | (61,836) |
corporate officers and
executive leadership
William F. Doyle
Executive Chairman
Asaf Danziger
Chief Executive Officer
Mike Ambrogi
Chief Operating Officer
Wilco Groenhuysen
Chief Financial Officer
Eilon Kirson, M.D., Ph.D.
Chief Science Officer and Head of Research and
Development
Todd Longsworth
General Counsel
Yoram Palti, M.D., Ph.D.
Founder
Pritesh Shah
Chief Commercial Officer
board of directors
William F. Doyle
Executive Chairman
Asaf Danziger
Chief Executive Officer
William Burkoth
Jeryl Hilleman
David T. Hung
Kinyip Gabriel Leung
Martin J. Madden
Sherilyn D. McCoy
Charles G. Phillips III
William A. Vernon

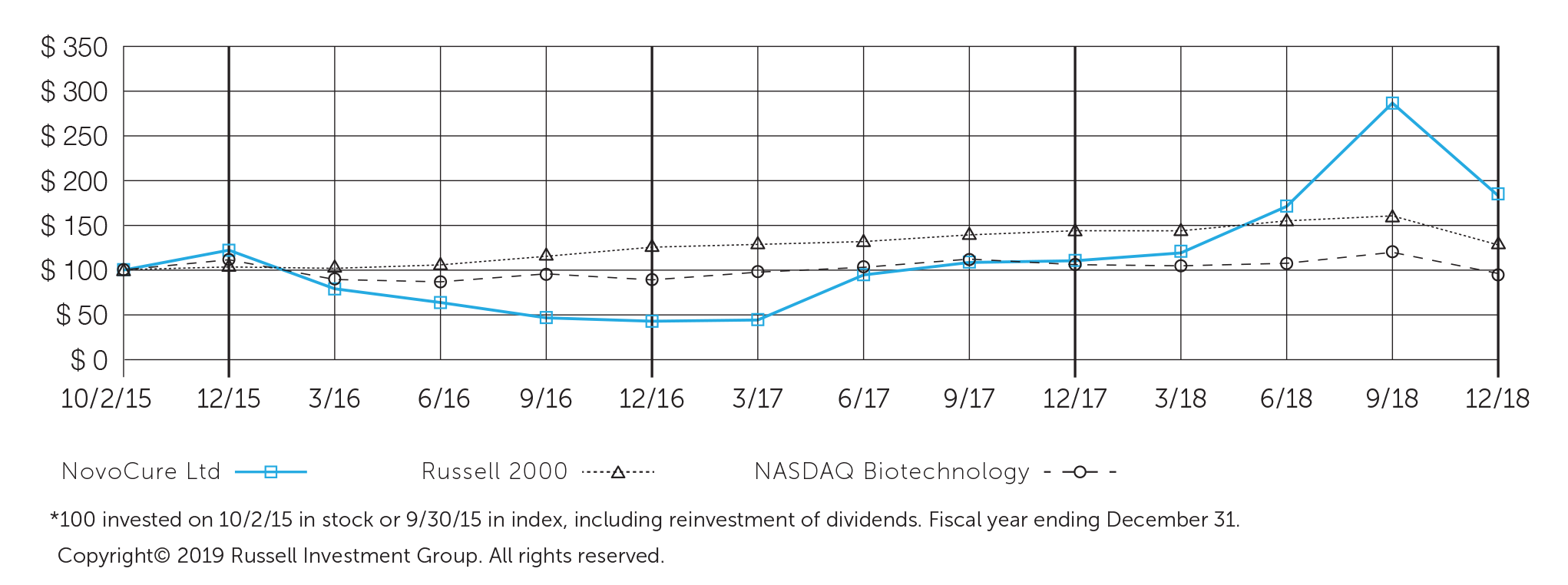
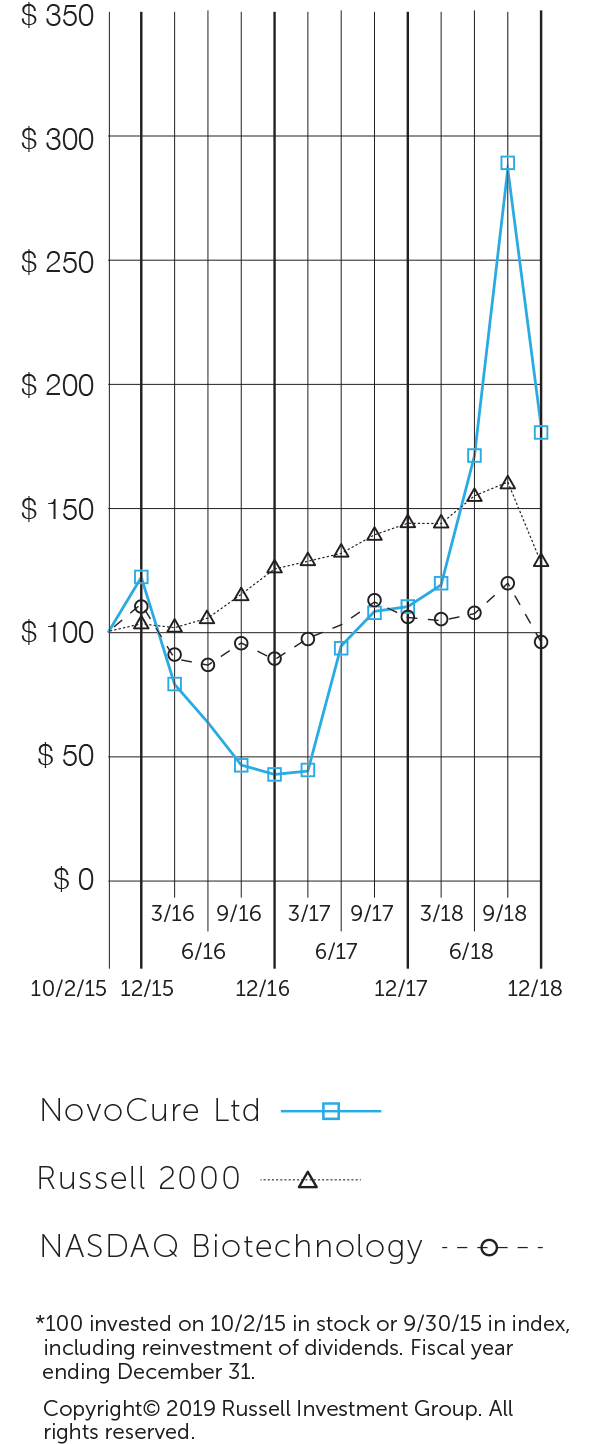
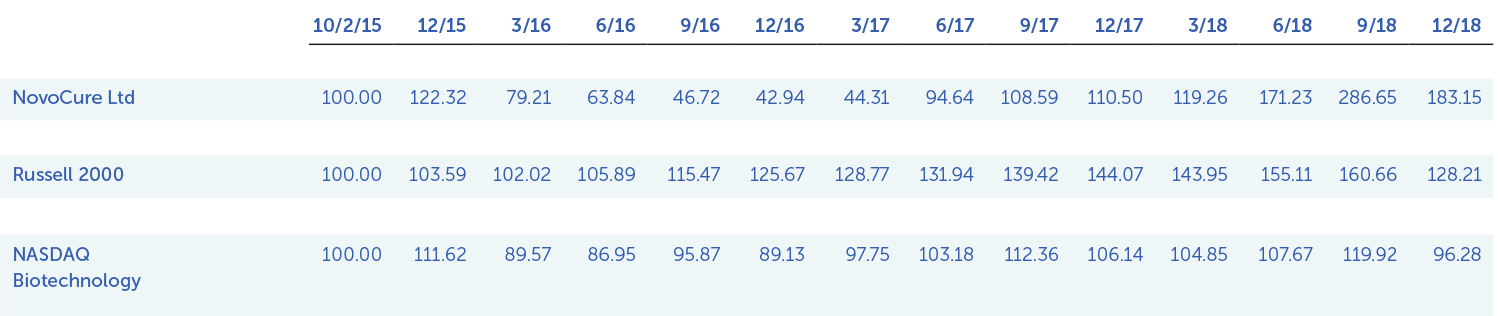
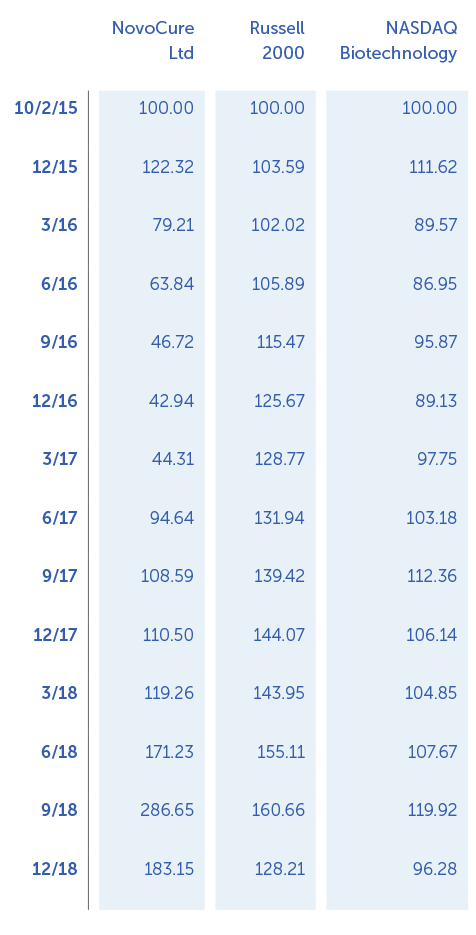
”With annual revenues appoaching $250 million, five indications in our late-stage pipeline and a strong balance sheet, we believe we are well-positioned to deliver significant long-term shareholder value.”
Chief Financial Officer